 03-20-2008, 09:49 AM
03-20-2008, 09:49 AM
|
#1
|
|
Unfrozen Caveman Lawyer
Join Date: Oct 2002
Location: Crowsnest Pass
|
 Organic Compounds Discovered On Extrasolar Planet
Organic Compounds Discovered On Extrasolar Planet
http://www.wired.com/science/space/n...8/03/exoplanet
Scientists using the Hubble Space Telescope have for the first time found the telltale signature of methane, an organic molecule, in the atmosphere of a planet outside our solar system.
Methane is one of the chemicals of life, an organic compound in the class of molecules containing carbon. However, no life is likely to exist on the large, gaseous planet known as HD 189733b. Its daily temperatures can reach 1,340 degrees Fahrenheit.
"These measurements are a dress rehearsal for future searches for life," said Mark Swain, a scientist at NASA's Jet Propulsion Laboratory and the lead author of a new study that appears in Nature tomorrow. "If we were able to detect [methane] on a more hospitable planet in the future, it would really be something exciting."
|

|

|
 03-20-2008, 10:01 AM
03-20-2008, 10:01 AM
|
#2
|
|
Franchise Player
Join Date: Jun 2004
Location: Vancouver
|
Can't we synthetically create organic compounds by adding certain molecules into a container and adding electricity? The major jump is from organic compounds to life.
|

|

|
 03-20-2008, 10:07 AM
03-20-2008, 10:07 AM
|
#3
|
|
Unfrozen Caveman Lawyer
Join Date: Oct 2002
Location: Crowsnest Pass
|

Quote:
Originally Posted by worth

Can't we synthetically create organic compounds by adding certain molecules into a container and adding electricity? The major jump is from organic compounds to life.
|
http://en.wikipedia.org/wiki/Miller-Urey_experiment
The Miller-Urey experiment (or Urey-Miller experiment) was an experiment that simulated hypothetical conditions present on the early Earth and tested for the occurrence of chemical evolution. Specifically, the experiment tested Oparin and Haldane's hypothesis that conditions on the primitive Earth favored chemical reactions that synthesized organic compounds from inorganic precursors. Considered to be the classic experiment on the origin of life, it was conducted in 1953 by Stanley L. Miller and Harold C. Urey at the University of Chicago.
At the end of one week of continuous operation Miller and Urey observed that as much as 10-15% of the carbon within the system was now in the form of organic compounds. Two percent of the carbon had formed amino acids, including 13 of the 22 that are used to make proteins in living cells, with glycine as the most abundant. Sugars, lipids, and some of the building blocks for nucleic acids were also formed. Nucleic acids (DNA, RNA) themselves were not formed. As observed in all consequent experiments, both left-handed (L) and right-handed (D) optical isomers were created in a racemic mixture.
http://www.sciam.com/article.cfm?id=...iment-repeated
Last edited by troutman; 03-20-2008 at 10:11 AM.
|

|

|
 03-20-2008, 10:18 AM
03-20-2008, 10:18 AM
|
#4
|
|
#1 Goaltender
Join Date: Nov 2005
Location: An all-inclusive.
|
Quote:
Originally Posted by troutman

As observed in all consequent experiments, both left-handed (L) and right-handed (D) optical isomers were created in a racemic mixture.
|
I just want to point out that this last statement is the really hard part. Every naturally occurring building block of life is only one optical isomer. Getting a racemic mixture means that you have a 50:50 mix of each optical isomer (they are mirror images of each other). Nobody knows where this chirality (that's what this is called) came from but it is really neat either way. Synthesizing one optical isomer over the other one is extraordinarily difficult.
|

|

|
 03-20-2008, 10:21 AM
03-20-2008, 10:21 AM
|
#5
|
|
Unfrozen Caveman Lawyer
Join Date: Oct 2002
Location: Crowsnest Pass
|
This news could impact the Drake Equation (increasing ne):
http://en.wikipedia.org/wiki/Drake_equation
The Drake equation states that:
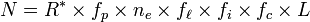 where:
N is the number of civilizations in our galaxy with which we might hope to be able to communicate;
and
R* is the average rate of star formation in our galaxy
fp is the fraction of those stars that have planets
ne is the average number of planets that can potentially support life per star that has planets
fℓ is the fraction of the above that actually go on to develop life at some point
fi is the fraction of the above that actually go on to develop intelligent life
fc is the fraction of civilizations that develop a technology that releases detectable signs of their existence into space
L is the length of time such civilizations release detectable signals into space.
where:
N is the number of civilizations in our galaxy with which we might hope to be able to communicate;
and
R* is the average rate of star formation in our galaxy
fp is the fraction of those stars that have planets
ne is the average number of planets that can potentially support life per star that has planets
fℓ is the fraction of the above that actually go on to develop life at some point
fi is the fraction of the above that actually go on to develop intelligent life
fc is the fraction of civilizations that develop a technology that releases detectable signs of their existence into space
L is the length of time such civilizations release detectable signals into space.
Last edited by troutman; 03-20-2008 at 10:29 AM.
|

|

|
 03-20-2008, 10:26 AM
03-20-2008, 10:26 AM
|
#6
|
|
Norm!
|
OH Zeldar can you point that thing away from me, thats disgusting.
__________________
My name is Ozymandias, King of Kings;
Look on my Works, ye Mighty, and despair!
|

|

|
 03-20-2008, 10:35 AM
03-20-2008, 10:35 AM
|
#7
|
|
Franchise Player
Join Date: Jul 2003
Location: In my office, at the Ministry of Awesome!
|
You know what I say?
I say to hell with our new Methane Based Estrasolar overlords.
This is our planet, they can go Fata themselves.
__________________
THE SHANTZ WILL RISE AGAIN.
 <-----Check the Badge bitches. You want some Awesome, you come to me!
|

|

|
 03-20-2008, 10:57 AM
03-20-2008, 10:57 AM
|
#8
|
|
Referee
Join Date: Jan 2005
Location: Over the hill
|
Quote:
Originally Posted by troutman

This news could impact the Drake Equation (increasing ne):
http://en.wikipedia.org/wiki/Drake_equation
The Drake equation states that:
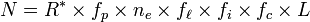 where:
N is the number of civilizations in our galaxy with which we might hope to be able to communicate;
and
R* is the average rate of star formation in our galaxy
fp is the fraction of those stars that have planets
ne is the average number of planets that can potentially support life per star that has planets
fℓ is the fraction of the above that actually go on to develop life at some point
fi is the fraction of the above that actually go on to develop intelligent life
fc is the fraction of civilizations that develop a technology that releases detectable signs of their existence into space
L is the length of time such civilizations release detectable signals into space.
where:
N is the number of civilizations in our galaxy with which we might hope to be able to communicate;
and
R* is the average rate of star formation in our galaxy
fp is the fraction of those stars that have planets
ne is the average number of planets that can potentially support life per star that has planets
fℓ is the fraction of the above that actually go on to develop life at some point
fi is the fraction of the above that actually go on to develop intelligent life
fc is the fraction of civilizations that develop a technology that releases detectable signs of their existence into space
L is the length of time such civilizations release detectable signals into space. |
I think I just sprained my amygdala. 
|

|

|
 03-20-2008, 01:21 PM
03-20-2008, 01:21 PM
|
#9
|
|
Franchise Player
|
Quote:
Originally Posted by worth

Can't we synthetically create organic compounds by adding certain molecules into a container and adding electricity? The major jump is from organic compounds to life.
|
As shown yes.
There are also experiments showing that amino acids can be formed in relatively short time at various temperatures (even as cold as liquid nitrogen if I remember correctly) from cyanide and ammonia sealed in a tube. Might have been something else as well but I can't remember.
|

|

|
 Posting Rules
Posting Rules
|
You may not post new threads
You may not post replies
You may not post attachments
You may not edit your posts
HTML code is Off
|
|
|
All times are GMT -6. The time now is 12:40 AM.
|
|

